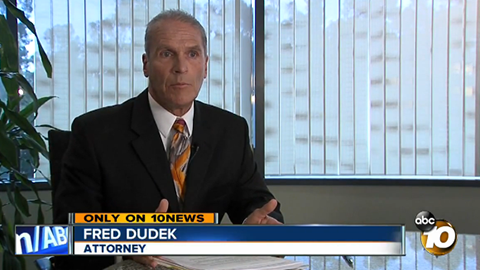
Manufacturing errors in the pharmaceutical industry are a real danger. Drug recalls are fairly common in the United States, and usually due to problems with the dosage on the label or adverse reactions after more people use the drug.
The Archives of Internal Medicine recently released a study examining FDA reports that concluded in the last eight years, in approximately 20 percent of Class 1 recalls, the FDA did not issue public notifications. When these drugs are determined to be defective or dangerous, the FDA is supposed to notify health care providers through either the Recall Alert System or MedWatch. The report indicated the FDA also fails to give priority to the most serious cases. According to study author Joshua Gagne, an epidemiologist at Brigham And Women’s Hospital, health care providers often lack vital details to appropriately respond to the defective drugs.
“[Regulators] need to be better in alerting the public to the most dangerous recalled drugs,” said Gagne, who suggests using new tracking technology on medications. “Thus, when a recall of a specific drug occurs, the pharmacy can identify all patients who have received that drug, but cannot identify which patients received the specifically recalled lots.”
If you or a loved one has been injured by a dangerous or defective drug, contact us today for a free consultation.
Bonnici Law Group, APC—San Diego injury attorney.





















 1620 5th Avenue
1620 5th Avenue 1620 5th Avenue
1620 5th Avenue