The Triad Group has issued a voluntary national pharmaceutical recall of alcohol prep pads that may be contaminated with Bacillus Cereus, a harmful and potentially deadly bacterium. Poisoning from the pads has caused least one known death.
Doctors pronounced two-year-old Harrison Kothari dead on December 1, 2010 from organ failure. Harrison was recovering from surgery—a cyst removal—at Children’s Memorial Hermann Hospital in Houston at the time of his death. Hospital staff allegedly used the recalled to clean a drain in Harrison’s spine during his hospitalization, according to MSNBC.com.
“They had no explanation as to how he contracted it,” said Sandra Kothari, Harrison’s mother. “They know it’s rare in the hospital.”
“These wipes were used in his care every single day, multiple times a day,” says Shanoop Kothari, Harrison’s father.
“We’re confident that’s the cause. There was no other explanation that made any sort of sense,” Shanoop Kothari said. “He contracted a very rare bacteria. These swatches were tainted with that bacteria.”
The Kotharis have filed a product liability lawsuit against the Triad Group. According to MSNBC, over 100 people have reported potential infections from the wipes.
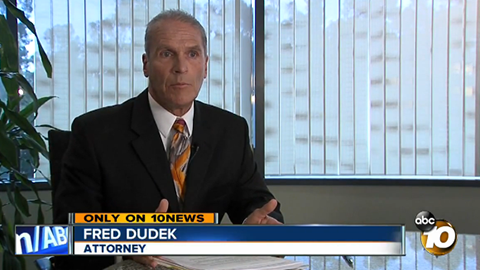
Joe Postich, a 55 year-old ironworker from Kentucky has also filed a product liability lawsuit against the Triad Group for $30 million. Postich claims the bacteria from the prep pads led to an infection that required him to have open-heart surgery and is now permanently disabled.
According to MSNBC, the FDA was aware of possible issues with the sterilization process at Triad’s Hartland, Wisconsin plant—which produces millions of the prep pads—as early as July 2009. The FDA raised questions about Triad’s sterilization process. They reported seeing worn and broken equipment during an inspection, however, the FDA issued to warnings to Triad.
The FDA conducted inspections of Triad’s Wisconsin plant in July 2009, and again in April and May of 2010. “Neither of those two inspections revealed information or evidence that allowed the agency to make conclusions that there was an imminent health hazard,” said Michael Rogers, acting director of the Office of Regional Operations for the FDA.
Once Triad notified the FDA of the possible contamination, the FDA “did everything correctly”, according to FDA representative Christopher Kelly. On an FDA document from January 7, it reads: “Sterile alcohol prep pads were found to be contaminated with…organisms and were released for shipment after the confirmation of the results.”
“It was reported to our office on a Saturday and we were in that facility on Monday,” Rogers said. “It is a huge recall that frankly would not have happened without the FDA’s inspection. What the recall achieves is to remove the product from the marketplace.”
Even with the recall, it is impossible to remove all of the pads from people’s homes and hospitals.
“People buy alcohol pads and they last a long time in your bathroom,” Sandra Kothari said. “They’re sitting there now. I wouldn’t want any other mother to go through what I’ve gone through.”





















 1620 5th Avenue
1620 5th Avenue 1620 5th Avenue
1620 5th Avenue