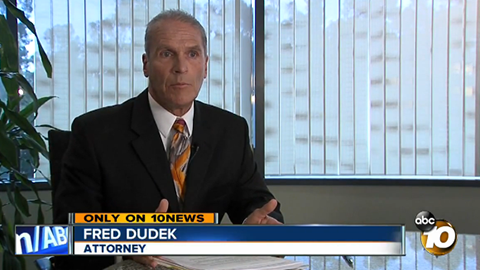
Medication shipped by the New England Compounding Center (NECC) recently was contaminated with fungus, causing a fungal meningitis outbreak causing 31 deaths in 17 states. In October, the Food and Drug Administration (FDA) investigated the conditions at NECC following reports from the Massachusetts Department of Health.
The Centers for Disease Control and Prevention (CDC) have reported more than 400 cases of meningitis linked to the medication, which was an injectable steroid painkiller. Many of the patients using the drug suffered from back pain and other types of joint pain.
The FDA and Massachusetts Department of Health have both reported unsanitary conditions at the NECC facility that may have contributed to the contamination. Federal investigators are looking into other medicines produced by the company to see if they may be contaminated as well.
NECC has a responsibility to the public to keep its facility sanitary to prevent outbreaks like this. For more information on pharmaceutical litigation, please visit our website. Contact our firm for a free consultation if you or a loved one has been injured or killed by a defective drug.
Bonnici Law Group, APC—San Diego injury lawyer.





















 1620 5th Avenue
1620 5th Avenue 1620 5th Avenue
1620 5th Avenue